Research
What We Do
(Possible graduate student projects listed)
Research Focus 1 (non-coding RNAs and the kidney)
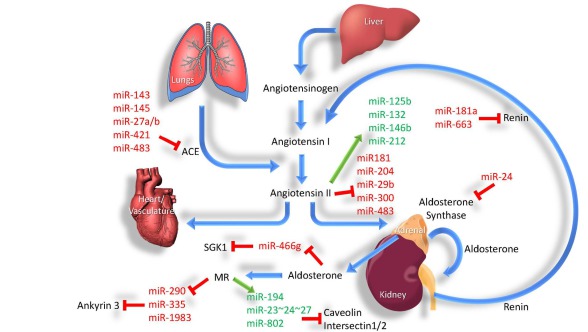
MicroRNAs in hormonal regulation of sodium transport in the kidney
MicroRNAs and sex differences in kidney disease
We are investigating the regulation of non-coding RNAs including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) by several hormones. We use a range of techniques to investigate the effect of steroid hormones (estrogen and aldosterone) on miRNA regulation in the kidney, and identify new protein targets that alter ion transport.
Graduate Student Projects
1) miRNA knockout/gain-of-function mice and sodium regulation in the kidney.
2) Sex differences in miRNA regulation by aldosterone.
3) Analysis of DeepSeq/RNASeq data to identify hormonally regulated miRNAs and lncRNAs in the kidney.
4) Physiological characterization of miR targets in aldosterone-mediated ENaC regulation.
5) miRNA feedback regulation of the mineralocorticoid receptor.
6) Role of insulin in miRNA regulation in the distal nephron, links to diabetes.
7) Estrogen regulation of miRNAs.
8) Sex differences in kidney injury, and links to miRNAs
Research Focus 2
Regulation of microRNAs by long non-coding RNA sponges
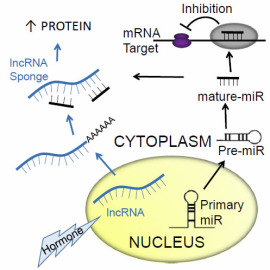
A role for microRNAs (miRs) has been established in growth, development, physiology and disease. Almost 2000 human miRs have been identified, and they act primarily to repress protein expression by binding to the untranslated region of mRNA to prevent protein translation and accelerate mRNA degradation (see schematic below). Far less studied are the long-ncRNAs (lncRNA) > 200bp in length. Several functions have been attributed to lncRNAs. They are able to interact with proteins, DNA and RNA in both the cytoplasm and nucleus to influence gene expression, translation or alter protein function. These studies aim to investigate whether lncRNA expression is regulated by hormonal signals, and if they scavenge smaller RNA species as an additional layer of gene regulation. Studies will focus on the idea that lncRNAs can act as miR sponges as a modifier of miR function.
Graduate Student Projects
1) Deep sequencing/microarray and computational approaches to predict and validate lncRNA-miR interactions.
2) Interrupting hormonal signaling by altering lncRNA expression.
3) Using tagged miRs as bait to pull out lncRNA interactors (by deep-seq and PCR)
4) Using lncRNAs to modify hormonal responses in cells
Research Focus 3
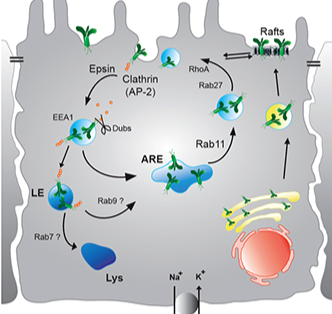
Regulation of the Epithelial Sodium Channel (ENaC) in kidney epithelia
The regulation of ENaC by trafficking and recycling mechanisms is investigated. We use CRISPR edited epithelial cell lines to understand the role of trafficking and scaffold proteins in ENaC trafficking. In the distal kidney nephron upregulation of the channel is associated with volume expansion and hypertension. Sex differnces in blood pressure regulation may be due to cross-talk between the mineralocorticoid and estrogen receptor signaling cascades and are being investigated.
Graduate Student Projects
1) The interplay between estrogen and aldosterone signaling in kidney epithelia
2) Cytoskeletal and motor proteins and ENaC trafficking
3) Disease-linked mutations in ENaC that cause hypertension